Transcatheter ViV procedures are becoming more common since they are less invasive than a surgical aortic valve (SAV) replacement which involves open heart surgery.7-8 These patients need a solution that will help to restore their healthy heart function. However, commercially available transcatheter aortic valves create a tradeoff between coronary access for future interventions and hemodynamics for better quality of life.9




DurAVR® INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE. EU: Exclusively for clinical investigations.
US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.
The DurAVR® THV System
The DurAVR® Transcatheter Heart Valve (THV) System has delivered paradigm-shifting hemodynamic performance#, combined with the deliverability performance of a balloon-expandable system. The System includes the DurAVR® THV, the ADAPT® anti-calcification tissue, and the ComASUR® Delivery System.
A balloon expandable valve with self-expanding hemodynamics is like the structural heart holy grail.

Dr Michael Reardon
Professor of Cardiothoracic Surgery, Allison Family Distinguished Chair of Cardiovascular Research Methodist DeBakey Heart & Vascular Center
The hemodynamics are so fantastic yet it has the ease, reliability, and accuracy of the balloon expandable valve … it combines all those things in a really well-designed valve.

Dr Rebecca T. Hahn
Director of Interventional Echocardiography at Columbia University
With DurAVR® we are seeing clinical results that look like a pre-disease state, with excellent hemodynamic performance and good laminar flow. In addition, it is incredibly easy to use.

Dr Chris Meduri
Interventional Cardiologist at Karolinska Institut and Anteris Chief Medical Officer
- slide-one
- slide-two
- slide-three

-
DurAVR® Transcatheter Heart Valve
The DurAVR® Transcatheter Heart Valve (THV) is a novel biomimetic valve made from a single piece of native-shaped tissue. It is designed to mimic the performance of a pre-disease human aortic valve.
-
Single-piece, Biomimetic Design
A single piece of ADAPT® tissue is molded to form native-shaped leaflets, designed to mimic the performance of a healthy aortic valve.
-
Coronary Access
Large open-cell geometry and short frame height designed to allow for access to the coronary ostia for future coronary interventions.
-
Balloon-Expandable Frame
Cobalt chromium frame expands with balloon inflation and is designed for controlled valve deployment and placement.
-
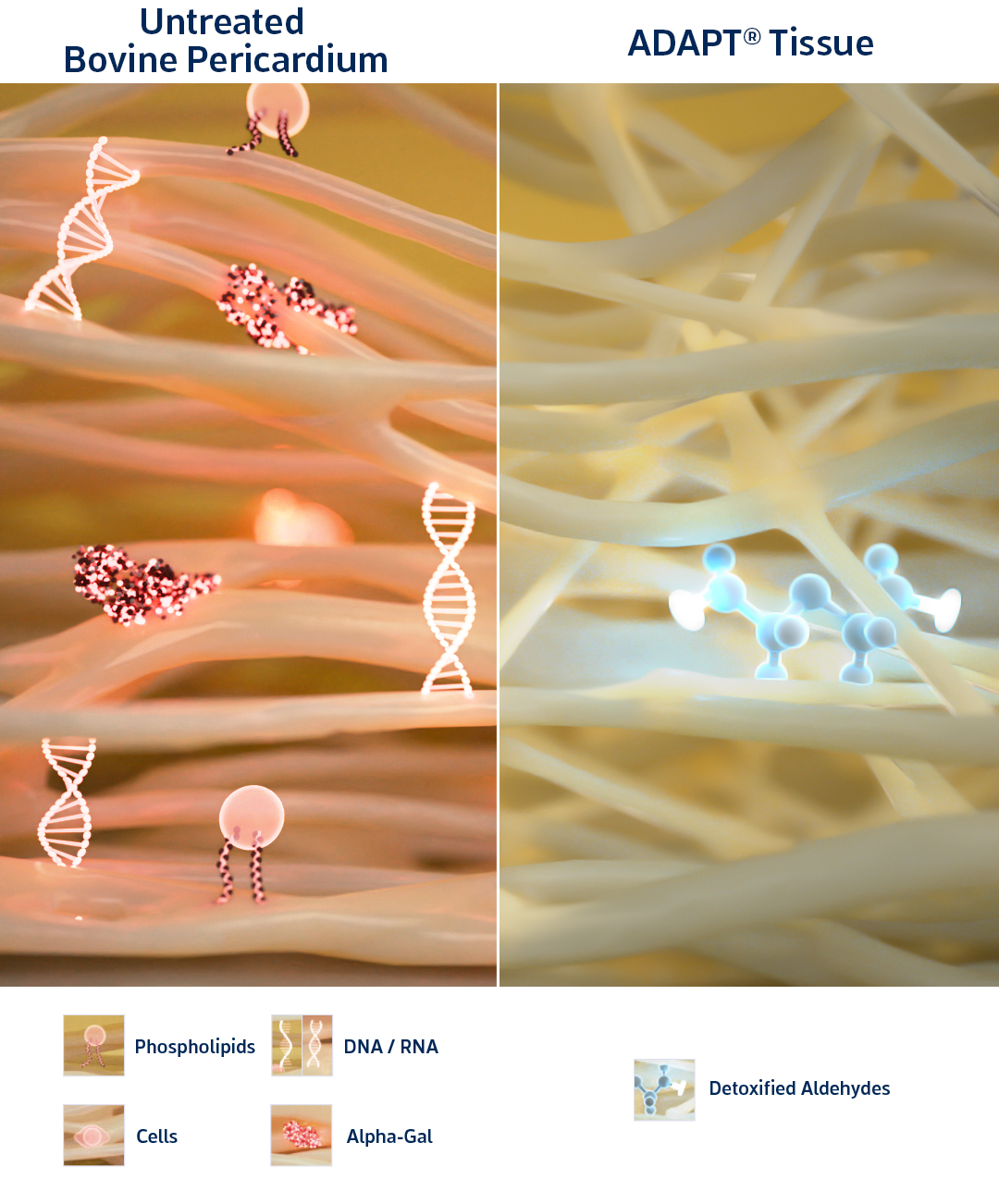
ADAPT® Tissue Process
The tissue is a patented bovine tissue that has been clinically proven to be calcium-free for up to 10 years1 and has been distributed for use in over 55,000 patients globally (as a cardiac and vascular patch).2 ADAPT® tissue is moldable, allowing it to be shaped for use in the DurAVR® heart valve.
ADAPT® tissue is designed for:
-
Anti-calcification
Undetectable levels of free aldehydes,2 a main driver of calcification-mediated valve deterioration3
-
Anti-fibrosis
The ADAPT® process is designed to remove xenogeneic pro-inflammatory factors that can lead to fibrosis (e.g., remnant DNA/RNA, phospholipids, alpha-gal epitopes).4-6
-
Reduced Leaflet Stressors
Moldable, pliable material allows for long coaptation to minimize leaflet pinwheeling and fluttering, thereby reducing the risk of valve deterioration.2
-
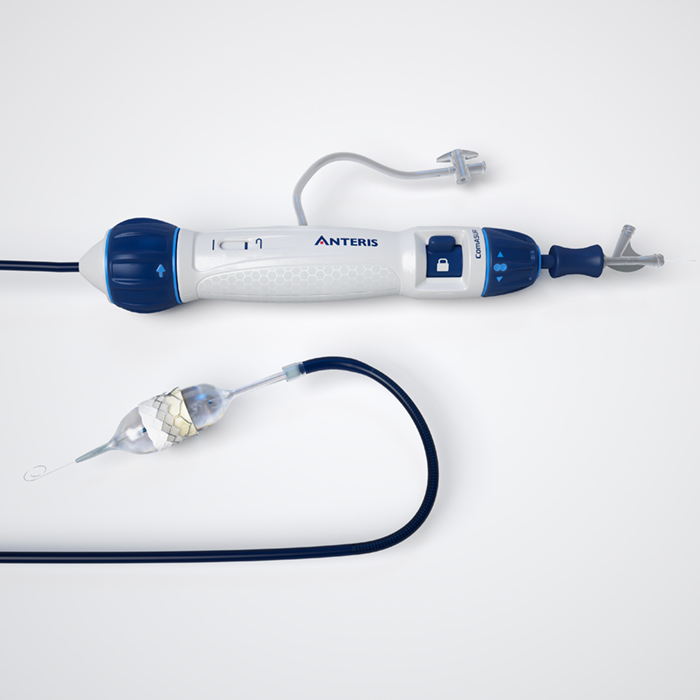
ComASUR® Delivery System
The ComASUR® Delivery System is designed for controlled deployment and accurate placement of the balloon-expandable DurAVR® THV. The ComASUR® Delivery System is designed to achieve ideal valve positioning through precise alignment with the heart’s native commissures.
-
Balloon-Expandable Deployment
Familiar procedural steps designed to allow controlled expansion and predictable, reliable placement
-
Precise Commissure Alignment
Patented design helps achieve optimal alignment with the native commissures during deployment
-
Distal Leading-Edge Protector
Flared distal end in which crimped valve is tucked creates additional protection during navigation
-
Steerable Catheter
Designed to ease navigation through the aortic arch
-
Expandable Sheath
14Fr expandable sheath for vessel access
SPECIAL USE CASES
Valve-in-Valve
Transcatheter Valve-in-Valve (ViV) replacement is performed by implanting a transcatheter heart valve within a previously implanted bioprosthetic aortic valve that is failing.
The DurAVR® transcatheter aortic valve (TAV) has a short frame design for easier coronary access, and its hemodynamics may make it the valve of choice for future ViV procedures. The DurAVR® valve has been successfully used in several TAV-in-SAV procedures globally through special access / compassionate use programs.
Paradigm-shifting Hemodynamic Performance
DurAVR® THV has delivered paradigm-shifting hemodynamic performance, measured by Effective Orifice Area (EOA), Mean Pressure Gradient (MPG) and Doppler Velocity Index (DVI).




Early Feasibility Study Data | 30-Day TTE Data, n=15, mean annulus: 22.7 mm | Presented at EuroPCR 2024
References
# Cavalcante J. Biomimetic Design Restores Flow and Hemodynamics and Leads to Significant LV Mass Regression: update from First-in-Human (FIH) Study with novel DurAVR™ Transcatheter Heart Valve. Oral Presentation at: New York Valves; June 2024; New York, New York.
† Lim KH, Candra J, Yeo JH, and Durán C, Flat or curved pericardial aortic valve cusps: A finite element study. The Journal of Heart Valve Disease, 2004;13(5): 792-7.
Anteris Data on File.
- Neethling W, Rea A, Forster G, Bhirangi K. Performance of the ADAPT-Treated CardioCel® Scaffold in Pediatric Patients With Congenital Cardiac Anomalies: Medium to Long-Term Outcomes, Front Pediatr, 2020;8:198
- Anteris Data on File
- Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, H. S. Kruth HS, Zand R, and Levy RJ, Prevention of Bioprosthetic Heart Valve Calcification by Ethanol Preincubation. Circul. 1997;95(2):479-488.
- Côté N, Pibarot P, and Clavel MA, Incidence, risk factors, clinical impact, and management of bioprosthesis structural valve degeneration. Curr Opin Cardiol. 2017;32: 123-129.
- Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M, Klepetko W, Wolner E, and Ankersmit HJ. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35(1):17-23.
- Badylak SF, and Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2): 109-16.
- Mehta C. Comprehensive Study Examines Age-Specific Trends and Outcomes in SAVR and TAVR. Oral presentation at: Society of Thoracic Surgeons Annual Meeting (STS); Jan 2024; San Antonio, TX.
- Otto C, Bonow R, Carabello B, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circul, 2021;143:72–227. doi:10.1161/CIR.0000000000000923.
- Di Muro FM, Cirillo C, Esposito L, et al. Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications. J Clin Med. 2024 Jan 7;13(2):341. doi:10.3390/jcm13020341.



