Subscribe to stay up-to-date on features and data releases
By clicking Sign Up, you confirm that you have read and agree to our Privacy Policy.
DurAVR® INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE. EU: Exclusively for clinical investigations. US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.
Promising Hemodynamic Performance
DurAVR® THV has demonstrated promising hemodynamic results, measured by Effective Orifice Area (EOA), Mean Pressure Gradient (MPG) and Doppler Velocity Index (DVI), sustained to 1 year in small annuli.
Mean Annulus Diameter: 22.7 mm

DurAVR® THV has demonstrated restoration of physiologic flow dynamics
When compared to a healthy aortic valve using cardiac MRI, DurAVR® THV showed no significant difference in flow. In addition, baseline 4D cardiac MRI from the DurAVR® THV EFS study shows turbulent flow with helical and vortical flow patterns, while imagery taken 6 months post-DurAVR® THV implantation shows evidence of healthy laminar flow through the valve and into the ascending aorta. Cardiac MRI is considered the gold standard for assessing and quantifying valve flow dynamics.
DurAVR® THV has demonstrated a restorative effect on Left Ventricular Mass
Patients treated with DurAVR® THV have experienced significant left ventricular (LV) mass regression, measured on cardiac MRI. Matched analysis also confirmed that the LV mass after treatment with DurAVR® THV was comparable to levels seen in healthy controls. A higher LV mass prior to TAVR has been associated with higher death and rehospitalization five years post-procedure.3 Likewise, LV mass regression one year after TAVR has been associated with lower death and hospitalization rates to five years post-procedure.4
LV Mass Pre- and Post-DurAVR® THV
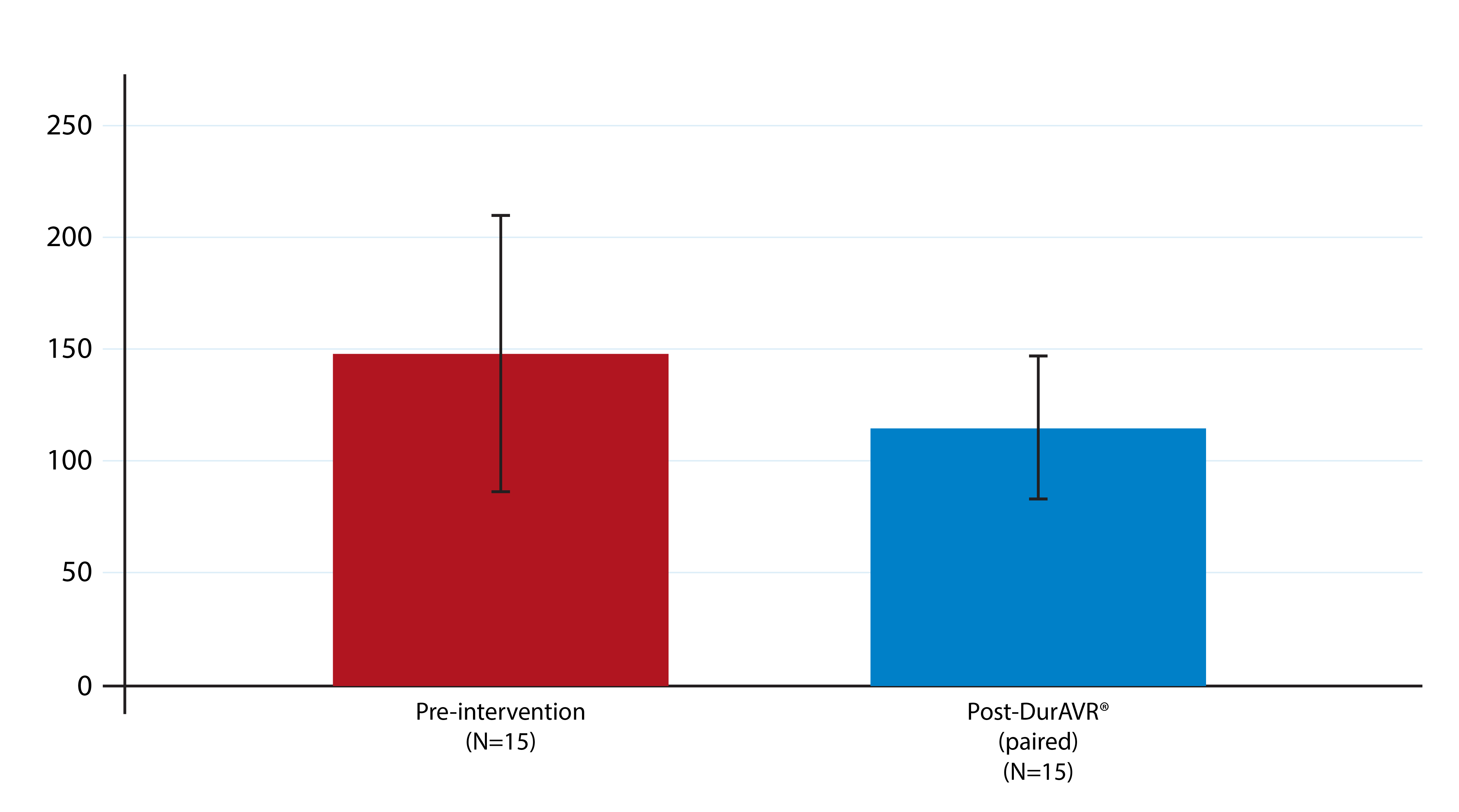
LV Mass
(g)
LV Mass Post-DurAVR® THV Matched to Healthy Controls
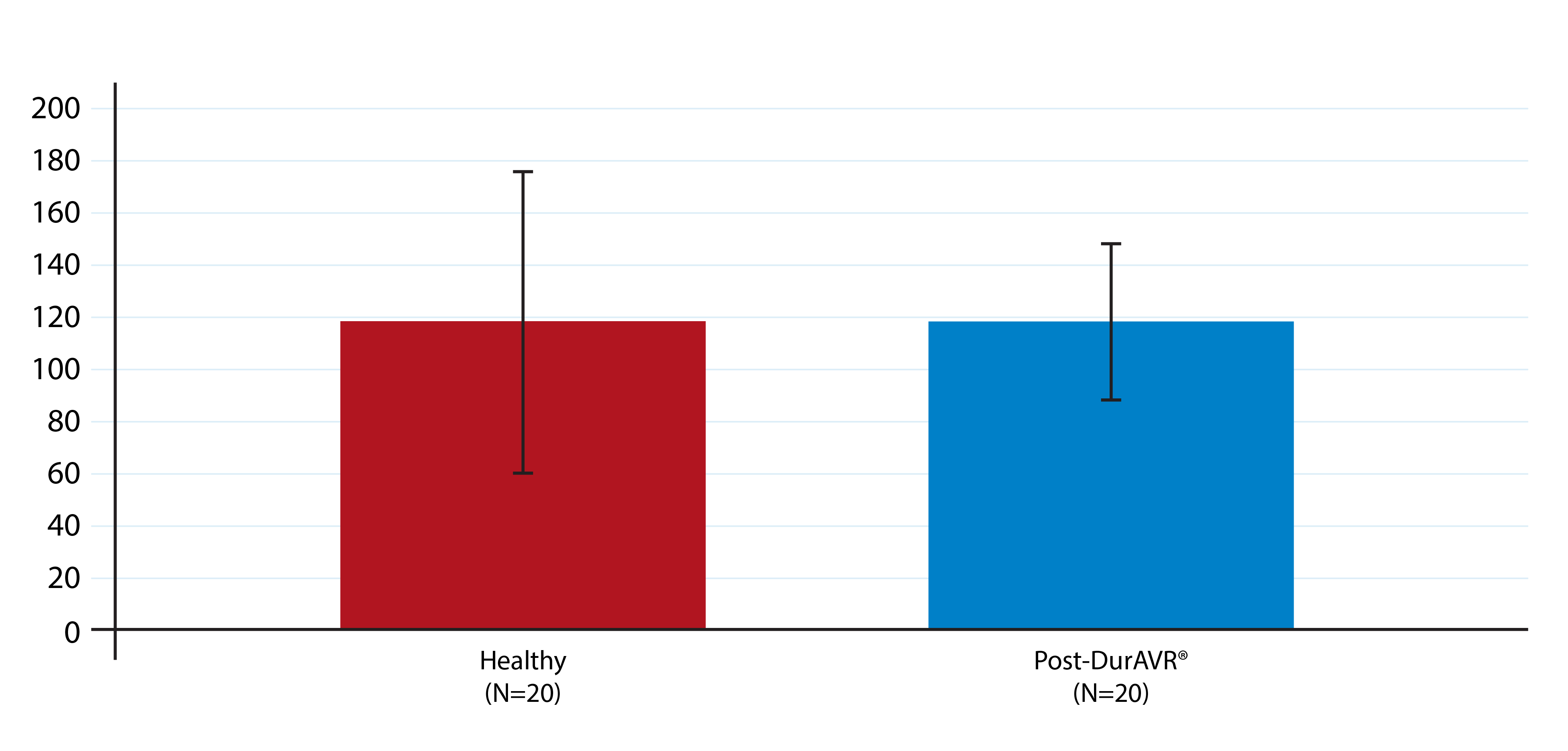
LV Mass
(g)
FIH Study, Presented at TCT 2024
DurAVR® THV Met Targeted Safety Endpoints
High implant success with a good safety profile was demonstrated.
| EFS & FIH Safety Data (n=65) | 30 Day | 1 Year* |
|---|---|---|
| All-cause Mortality** | 0% | 4.6% |
| CV Mortality | 0% | 0 |
| Disabling Stroke | 0% | 1.5% |
| Endocarditis*** | 0% | 1.5% |
| Permanent Pacemaker | 7.7% | 9.2% |
| Coronary Obstruction | 0% | 0% |
| Acute Kidney Injury stage 2/3 | 0% | 0% |
| Cardiovascular Hospitalizations | 3.1% | 6.2% |
Puri R. Oral Presentation at: Sydney Valves; March 2025; Sydney, Australia.
DurAVR® INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE. EU: Exclusively for clinical investigations.
US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.
No Moderate or Severe Paravalvular Leak

97% freedom from ≥ moderate prosthesis-patient mismatch1

Puri R. Oral Presentation at: Sydney Valves; March 2025; Sydney, Australia.
De Backer O. Oral Presentation at: PCR London Valves; Nov 2025; London, UK.
Hear the Latest Clinical Insights
Subscribe to stay up-to-date on features and data releases
By clicking Sign Up, you confirm that you have read and agree to our Privacy Policy.
DurAVR® INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE. EU: Exclusively for clinical investigations. US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.
Publications
References
- De Backer O. Outcomes of a Novel Biomimetic Balloon Expandable Valve in Small Aortic Annuli. Oral Presentation at: PCR London Valves; Nov 2025; London, UK.
- Puri R. DurAVR®: A Novel First-in-Class Biomimetic Transcatheter Aortic Valve 1-Year Performance. Oral Presentation at: Sydney Valves; March 2025; Sydney, Australia.
- Gonzales H, Douglas PS, Pibarot P, et al. Left Ventricular Hypertrophy and Clinical Outcomes Over 5 Years After TAVR: An Analysis of the PARTNER Trials and Registries. JACC Cardiovasc Interv. 2020;13(11):1329-1339. doi:10.1016/j. jcin.2020.03.011
- Chau K, Douglas P, Pibarot P, et al. Regression of left ventricular mass after transcatheter aortic valve replacement: The PARTNER trials and registries. JACC. 2020;75(19): 2446–2458. doi:10.1016/j.jacc.2020.03.042






