
December 03, 2025
Annual Meeting 2025
Anteris Technologies Vice Chairman and Chief Executive Officer, Wayne Paterson presents at the Annual Meeting held on December 3, 2025 (US CT) / December 4 (AEST) in Brisbane Australia.

December 03, 2025
Anteris Technologies Vice Chairman and Chief Executive Officer, Wayne Paterson presents at the Annual Meeting held on December 3, 2025 (US CT) / December 4 (AEST) in Brisbane Australia.

October 27, 2025
The PARADIGM Trial is the first head-to-head TAVR trial of its kind. The trial will move beyond conventional endpoints of safety, efficacy, and hemodynamic performance, aiming to also uncover the clinical impact of restoring physiologic flow patterns.
Subscribe to our newsletter and stay up to date on features and releases.
By subscribing you agree to with our Privacy Policy and provide consent to receive updates from our company.

Presented at London Valves and sponsored by Anteris, Dr. Miho Fukui’s research focuses on a new concept that uses a percentage in aortic valve area (AVA) reduction for determining disease severity, rather than applying the same AVA cutoffs to all patients despite different valve sizes.

At London Valves, Prof. Ole De Backer presented 30-day data from 100 small annuli patients treated with DurAVR® THV in the EMBARK and EFS studies.
Highlights of 30-Day Results for 100 Small Annuli Patients Implanted with the DurAVR® THV
*Prosthesis‐patient mismatch (PPM) happens when a prosthetic valve, after being implanted, doesn't have a large enough opening (EOA) to accommodate the patient's blood flow needs, based on their body size. The result is higher than expected gradients. PPM affects a significant proportion of transcatheter aortic valve (TAVR) patients, particularly patients with a small aortic annulus and has been associated with impaired long-term survival following surgical aortic valve replacement (SAVR).
**As defined in VARC-3.

Hear perspectives from Prof. Ole De Backer and Prof. Nicolas Van Mieghem about the recent DurAVR® THV data and the PARADIGM Trial in this 2-part discussion series.
Part 1 highlights the PARADIGM trial—the first all-comers, head-to-head TAVI trial and the first TAVI trial designed to evaluate post-procedure blood flow patterns using cardiac MRI. With participation from 80 centers across the US, Canada, and Europe, recent FDA IDE approval, and first enrollments already completed in Denmark in October, the PARADIGM Trial is expected to enroll quickly, and the data gathered will be used to support future commercial approval of the DurAVR® valve for patients across all risk categories.
Part 2 focuses on Prof. De Backer’s presentation of 30-day outcomes from 100 small annuli DurAVR® THV patients, providing early insights into clinical performance. DurAVR® THV demonstrated a favorable hemodynamic profile at 30 days, including 97% freedom from moderate or greater patient-prosthesis mismatch (PPM).1
1 De Backer O. Oral Presentation at: PCR London Valves; Nov 2025; London, UK.

Presented at TCT and sponsored by Anteris, Dr. Miho Fukui’s research focuses on a new concept that uses a percentage in aortic valve area (AVA) reduction for determining disease severity, rather than applying the same AVA cutoffs to all patients despite different valve sizes.

At TCT, Dr. Rishi Puri presented data from DurAVR® THV clinical experience, demonstrating a favorable hemodynamic profile for 37 patients sustained to 1-year follow-up.
One-year Results Highlights:

Anteris Technologies Global Corp. announces that on September 29, 2025, the Company held a Special Meeting of Stockholders (the Special Meeting) at which a quorum was present. The matters listed below were submitted to a vote of the Company’s stockholders at the Special Meeting through the solicitation of proxies. Detailed descriptions of the proposals are included in the Company’s definitive proxy statement on Schedule 14A filed with the Securities and Exchange Commission on August 18, 2025 (the “Proxy Statement”).
A total of 18,687,740 shares of the Company’s common stock were present at the Special Meeting in person, by virtual attendance, or by proxy, which represents approximately 51.8% of the shares of common stock outstanding as of August 11, 2025, which was the record date for the Special Meeting.
A recording of the meeting will be available at this link for 90 days after the meeting.
AS affects the valve, vessels, and myocardium. This webinar discusses how DurAVR® THV restores laminar flow, improving LV function and mass regression in AS patients, shifting focus from traditional metrics to whole-heart function.
Presented by: Drs. Martin B. Leon, Tsuyoshi Kaneko, Brian Lindman, and Jeffrey Popma.

In this whitepaper, we review the pathophysiology of aortic stenosis—including upstream and downstream consequences of turbulent flow—and offer hypotheses around the impacts of TAVR design on flow patterns and disease regression.
We will explore answers to questions like:

At Sydney Valves, Dr. Rishi Puri presented data from DurAVR® THV clinical experience, demonstrating a favorable hemodynamic profile for 37 patients sustained to 1-year follow-up.
One-year Results Highlights:
“The one-year data for DurAVR® THV continues to validate its groundbreaking hemodynamic performance, demonstrating sustained excellent effective orifice area (EOA) and low mean gradients. Most notably, this is the only transcatheter valve to show zero prosthesis-patient mismatch (PPM) in small annuli patients—an achievement that sets a new standard in TAVR. PPM is a well-established predictor of valve failure and disease progression, and eliminating it has profound implications for long-term patient outcomes. These results reinforce the transformative potential of DurAVR® as we move toward pivotal trials.” - Christopher Meduri, Anteris Chief Medical Officer

At TCT 2024, Dr. Amar Krishnaswamy presented the latest MRI data analyses which compared flow dynamics and left ventricular function between DurAVR® THV recipients and a matched healthy control group. Here were some of the key takeaways:
In this matched analysis, DurAVR® THV’s biomimetic design provides flow patterns similar to the healthy valve, as measured by flow displacement (FD) and flow reversal ratios (FRR).
LV mass decreased in patients treated with DurAVR® THV compared to baseline, suggesting an improvement in LV unloading.
In this matched analysis, DurAVR® THV restored left ventricle (LV) end-diastolic volume, LV end-systolic volume, and LV mass to similar to healthy controls.
At London Valves 2024, Dr. Pankaj Garg presented cutting-edge cardiac MRI analyses, which compared flow dynamics and LV function between DurAVR® THV recipients and a healthy control group. These promising data highlight the potential of DurAVR® THV to mimic the performance of a healthy aortic valve as a result of the valve's biomimetic design.

Drs. Amar Krishnaswamy, Nicolas Van Mieghem, and Bernard Prendergast review the latest DurAVR® THV MRI flow & LV mass data compared to a healthy control group, exploring the impact of valve design on laminar flow, disease regression, and patient outcomes.

The recording of our Annual General Meeting is now available for viewing. This comprehensive session includes detailed discussions, strategic updates, and future plans that underscore the continued growth and innovation at Anteris Technologies.
We invite all stakeholders, partners, and interested parties to watch this recording to stay informed about our latest developments and achievements. Your continued support and engagement are greatly appreciated.

We are pleased to announce new DurAVR® THV First-in-Human study data was presented at New York Valves by imaging expert Dr. João Cavalcante. “When we look at commercially available surgical or TAVR valves, we are still seeing abnormal flow patterns on cardiac MRI. The restoration of laminar flow, as we are seeing with this new DurAVR® THV, is a byproduct of the intrinsic valve design and novel technology, which might have positive downstream implications to the arteries and consequently to ventricle, and ultimately to the patients,” stated Dr. Cavalcante.

At CRT 2024, Dr Rishi Puri presented an update on DurAVR® THV. The biomimetic valve is showing consistently promising hemodynamic performance—measured by EOA, MPG, and DVI—from 30 days through 1 year. Learn about DurAVR® THV and view the full presentation on the EFS and FIH studies below.

At CRT 2024, Dr Thomas Waggoner presented 30-day data from the DurAVR® transcatheter heart valve (THV) Early Feasibility Study (EFS). The data substantiates the promising hemodynamic (blood flow) results reported to date with the DurAVR® THV, a new class of biomimetic THV designed to restore normal blood flow in aortic stenosis. The latest data includes 30-day results for all 28 patients treated in the First-In-Human Study (Cohorts 1-4) in addition to 30-day results for all 15 patients treated in the US Early Feasibility Study. View the full presentation below.
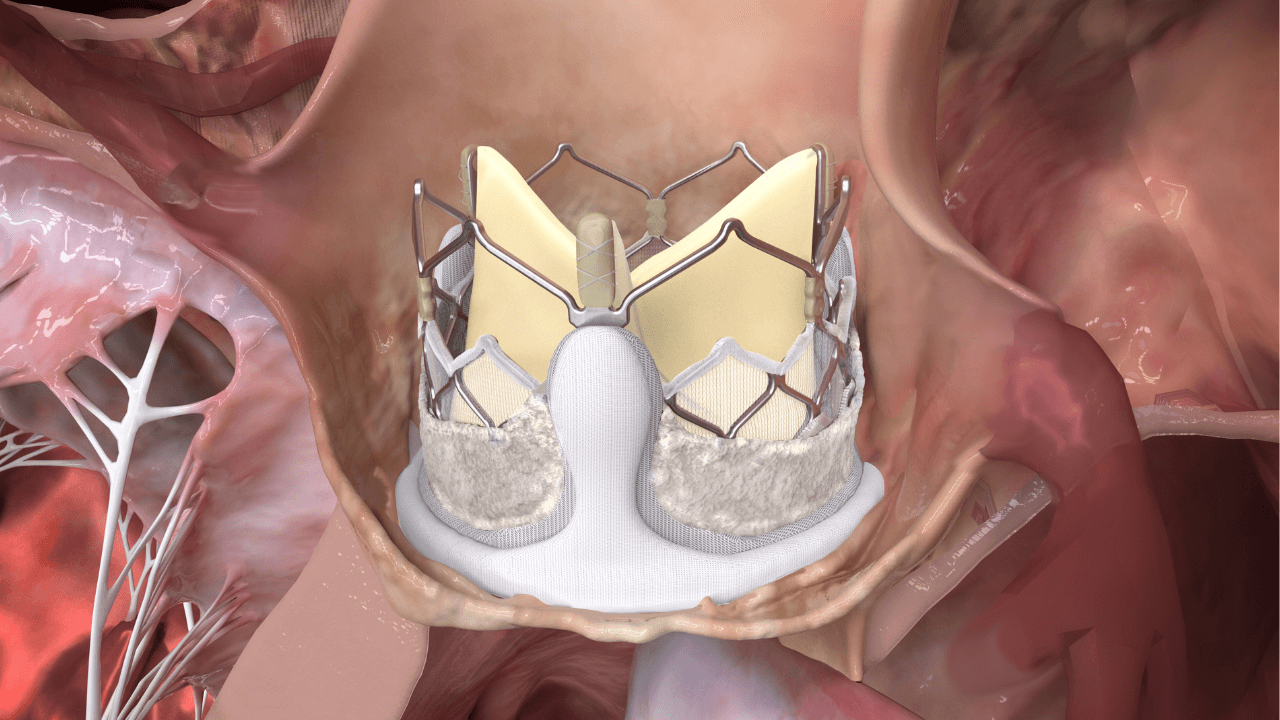
In this expert interview from PCR London Valves 2023, Dr Vinayak Bapat interviews Dr Anita Asgar regarding the challenges physicians face today with valve-in-valve (ViV) patients and the potential utility of DurAVR® for ViV procedures through case-based discussion. Learn about:

In this video, Drs Nicolas Van Mieghem, Christopher Meduri, and Azeem Latib discuss the biomimetic design of the DurAVR® transcatheter heart valve (THV) and its impact on hemodynamics and blood flow dynamics. Learn about:
View the 1-year data from the DurAVR® THV First-In-Human study presented at EuroPCR 2023 by Dr Susheel Kodali. Excellent hemodynamic results are sustained to 1 year.

At EuroPCR 2023, Drs Christopher Meduri, Susheel Kodali, and João Cavalcante discuss the changing TAVR landscape and the early safety and feasibility of a first new class of biomimetic transcatheter aortic valve – DurAVR® Transcatheter Heart Valve (THV).
Learn about:

At SCAI 2024, Dr Azeem Latib presented an update on the DurAVR® THV First-in-Human Study, including consistent hemodynamics and excellent safety profile through 1-year post-implantation.
Learn about:

Presented at the 2022 Annual General Meeting, Anteris Technologies CEO and director, Wayne Paterson, connects live to the Cath Lab in Tbilisi, Georgia for an update on the DurAVR® THV First-in-Human study with implanting physicians, Dr. Christopher Meduri and Dr. Vinayak Bapat. The physicians speak about the successful cases performed and the performance of the ComASUR® Delivery System. Recorded footage provides an inside look as healthcare professionals set up the Cath Lab operating room while setting up and performing the DurAVR® TAVR procedure.

Watch leading experts in the field of TAVR discuss the biomimetic design of the DurAVR® THV and its impact on hemodynamics and blood flow dynamics, presented by Drs. Christopher Meduri, Susheel Kodali, João Cavalcante, Michael Reardon, Nicolas Van Mieghem, Azeem Latib, Anita Asgar and Vinayak Bapat.
Key takeaways included:

Watch this physician panel and hear Drs Michael Reardon, Christopher Meduri, and Azeem Latib discuss the design of the DurAVR® Transcatheter Heart Valve and the paradigm-shifting hemodynamic results from the Early Feasibility Study (EFS).
Learn about:
At the 2023 PCR London Valves conference, Dr Azeem Latib presented 30-day data from the US Early Feasibility Study (EFS) for the DurAVR® Transcatheter Heart Valve (THV). The preliminary results 30 days post-procedure included data from 12 out of the 15 enrolled patients (three patients awaiting scheduling).

At London Valves 2022, Dr Christopher Meduri presented results from the DurAVR® THV First-in-Human study, providing preliminary echo, CT, and MRI evidence of improved hemodynamic and laminar flow characteristics associated with the DurAVR® THV System.
This prospective, non-randomized, single-arm, single-center study to evaluate the safety and feasibility of DurAVR® THV was conducted in a patient cohort of 13 subjects with severe symptomatic aortic stenosis including challenging anatomies such as Type 1 bicuspid and extreme leaflet calcium.

At London Valves 2022, Dr Pankaj Garg presented formal results of a comparative study to investigate aortic flow physiology by cardiac MRI in five patients who received a DurAVR® transcatheter aortic valve (first-in-human). The study compared DurAVR® THV to normal age-height-weight matched controls, other transcatheter aortic valve implants (TAVI) and patients who had surgical aortic valve replacement (SAVR).
At TCT 2023, Dr Stephanie Sellers presented DurAVR® THV bench study data for redo-TAVR. View the slides to see comparative hydrodynamic data with DurAVR® THV, which demonstrated superior hydrodynamic performance in redo-TAVR with ‘failed’ SAPIEN 3. DurAVR® THV also outperformed all other commercially available THVs with regard to hydrodynamics and pinwheeling.
At TCT 2023, Dr Azeem Latib presented the preliminary results from the DurAVR® THV US Early Feasibility Study. View the slides from the presentation to learn about the design of the biomimetic THV and see the paradigm-shifting hemodynamic results at discharge for all 15 enrolled patients.

See the latest DurAVR® THV First-in-Human (FIH) Study data presented by Dr Christopher Meduri at TCT 2023, showing sustained mean hemodynamic performance from all 20 patients at 6 months post-procedure.

Watch this video to hear from an expert panel of physicians, including Drs Martin Leon, Michael Reardon, Thomas Modine, Rebecca Hahn, and Christopher Meduri. The panel discusses:

See the latest data presented by Dr Christopher Meduri at TVT 2023, showing sustained haemodynamic performance to 1 year and highlighting our latest patient cohort showing best results to date at 30 days.

We are excited to share the promising results from a valve-in-valve (ViV) study led by Dr. Anita Asgar and recently presented at New York Valves. The data demonstrated restoration of hemodynamic performance in five complex cases in which patients underwent a DurAVR® Transcatheter Heart Valve implantation after their surgical replacement valves failed.
Dr. Asgar emphasized, “This data set of valve-in-valve patients highlights the impact of the DurAVR® valve biomimetic design by restoring these patients’ hemodynamics to the performance of their initial surgical valve and is very encouraging for the wave of valve-in-valve patients that are coming in the future.”