Healthy aortic valve vs DurAVR™ THV: No significant difference in flow (p>0.05)
FIH Study, Presented at New York Valves 2024.
Sign-Up for Clinical Data Updates
By clicking Sign Up, you confirm that you have read and agree to our Privacy Policy.
DurAVR™ INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE. EU: Exclusively for clinical investigations. US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.
Paradigm-shifting Hemodynamic Performance
Now with US EFS results, DurAVR™ THV delivers outstanding hemodynamic results, measured by Effective Orifice Area (EOA), Mean Pressure Gradient (MPG) and Doppler Velocity Index (DVI).
Mean Annulus: 22.73mm
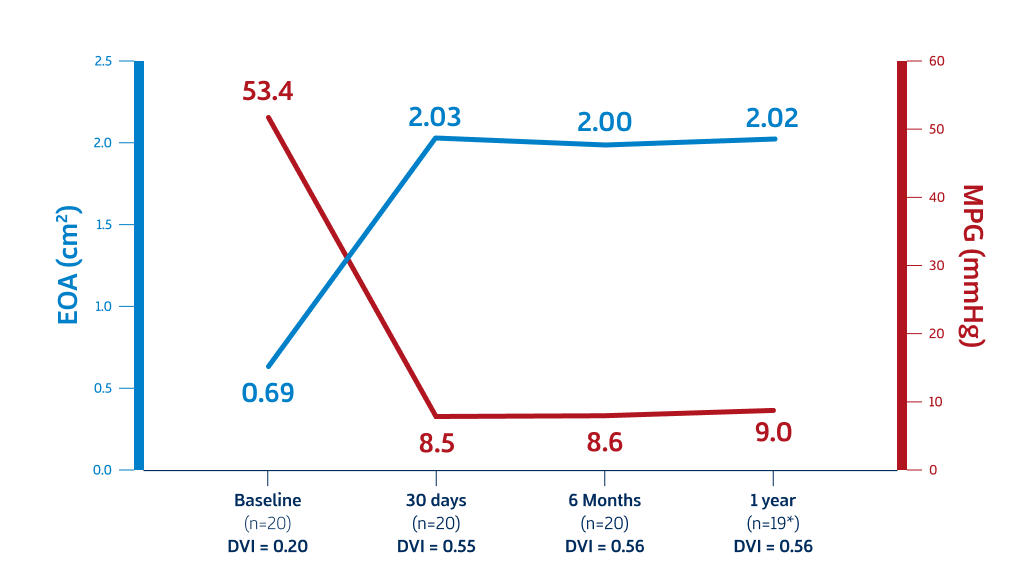
First‐In‐Human Data (1 Year)
Cavalcante J. Biomimetic Design Restores Flow and Hemodynamics and Leads to Significant LV Mass Regression: update from First-in-Human (FIH) Study with novel DurAVRTM Transcatheter Heart Valve. Oral Presentation at: New York Valves; June 2024; New York, New York.
*One subject died of a non-cardiac death before reaching 1-year follow-up.
Mean Annulus: 22.2mm
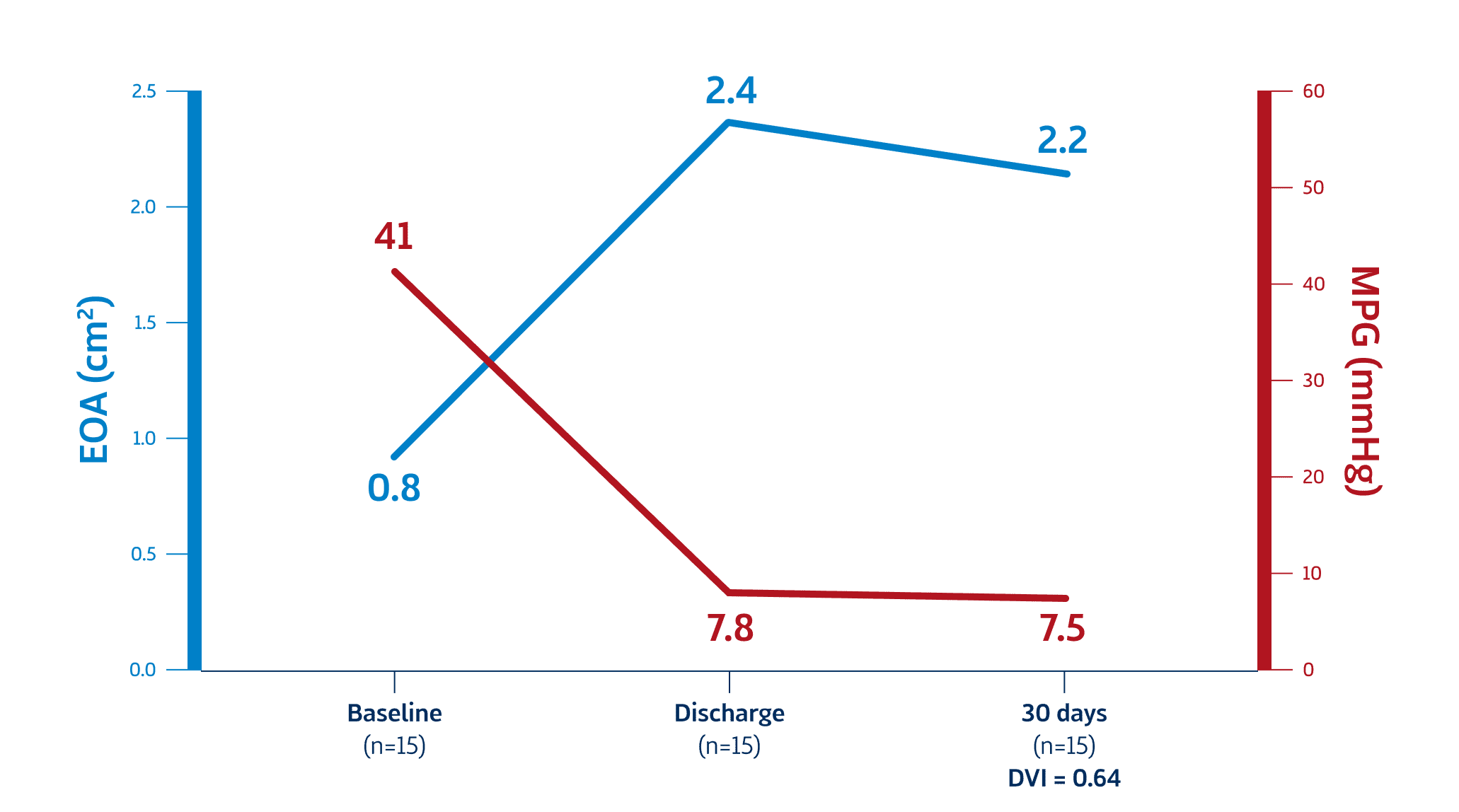
Early Feasibility Study Data
Meduri C. DurAVR™ biomimetic transcatheter heart valve: early feasibility and first-in-human trial update. Oral presentation at: EuroPCR Conference; May 2024; Paris, France.

DurAVR™ Met Targeted Safety Endpoints
High implant success with a good safety profile was demonstrated.
No valve-related adverse events occurred to 1-year follow-up
| EFS Safety Data | 30 Days (n=15) |
|---|---|
| Primary Safety Endpoints | |
| All-cause Mortality or Disabling Stroke | 0 |
| Secondary Safety Endpoints | |
| All-cause Mortality | 0 |
| Disabling Stroke | 0 |
| VARC-3 Type 2-4 Bleeding | 0 |
| Major Vascular or Structural Heart Complications | 0 |
| Acute Kidney Injury (AKI) Stage 3 or 4 | 0 |
| Moderate or Severe Aortic Regurgitation | 0 |
| New Permanent Pacemaker | 1 (6.7)* |
| Surgery or Intervention Related to the Device, including Aortic Valve Reintervention | 0 |
| FIH Safety Data | ≤30 Days (n=41) | >30 days (n=20) |
|---|---|---|
| Primary Safety Endpoints | ||
| All-cause Mortality | 0 | 1 (5)* |
| Myocardial Infarction | 0 | 0 |
| Disabling Stroke | 0 | 0 |
| Life-threatening Bleeding | 0 | 0 |
| Secondary Safety Endpoints | ||
| Valve-related Mortality | 0 | 0 |
| All Strokes | 1 (2.4)** | 0 |
| All Bleeding | 2 (4.9) | 0 |
| Major Access Site / Vascular Complications | 4 (9.8) | 0 |
| New Permanent Pacemaker | 2 (4.9)*** | 0 |
| Bioprosthetic Valve Dysfunction or SVD | 0 | 0 |
| Aortic Valve Reoperations/Reintervention | 0 | 0 |
| Acute Kidney Injury (AKI) | 0 | 0 |
| Major Paravalvular Leak (moderate or severe) | 0 | 0 |
EFS Study, Presented at EuroPCR 2024. FIH Study, Presented at EuroPCR 2024.
DurAVR™ INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE.
EU: Exclusively for clinical investigations.
US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.
Hear the Latest Clinical Insights
Sign-Up for Clinical Data Updates
By clicking Sign Up, you confirm that you have read and agree to our Privacy Policy.
DurAVR™ INVESTIGATIONAL USE ONLY. NOT AVAILABLE FOR COMMERCIAL SALE. EU: Exclusively for clinical investigations. US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.


